Development of a transgene-free gene editing system in European Pear
Author: Jessica Waite
Published: 2024
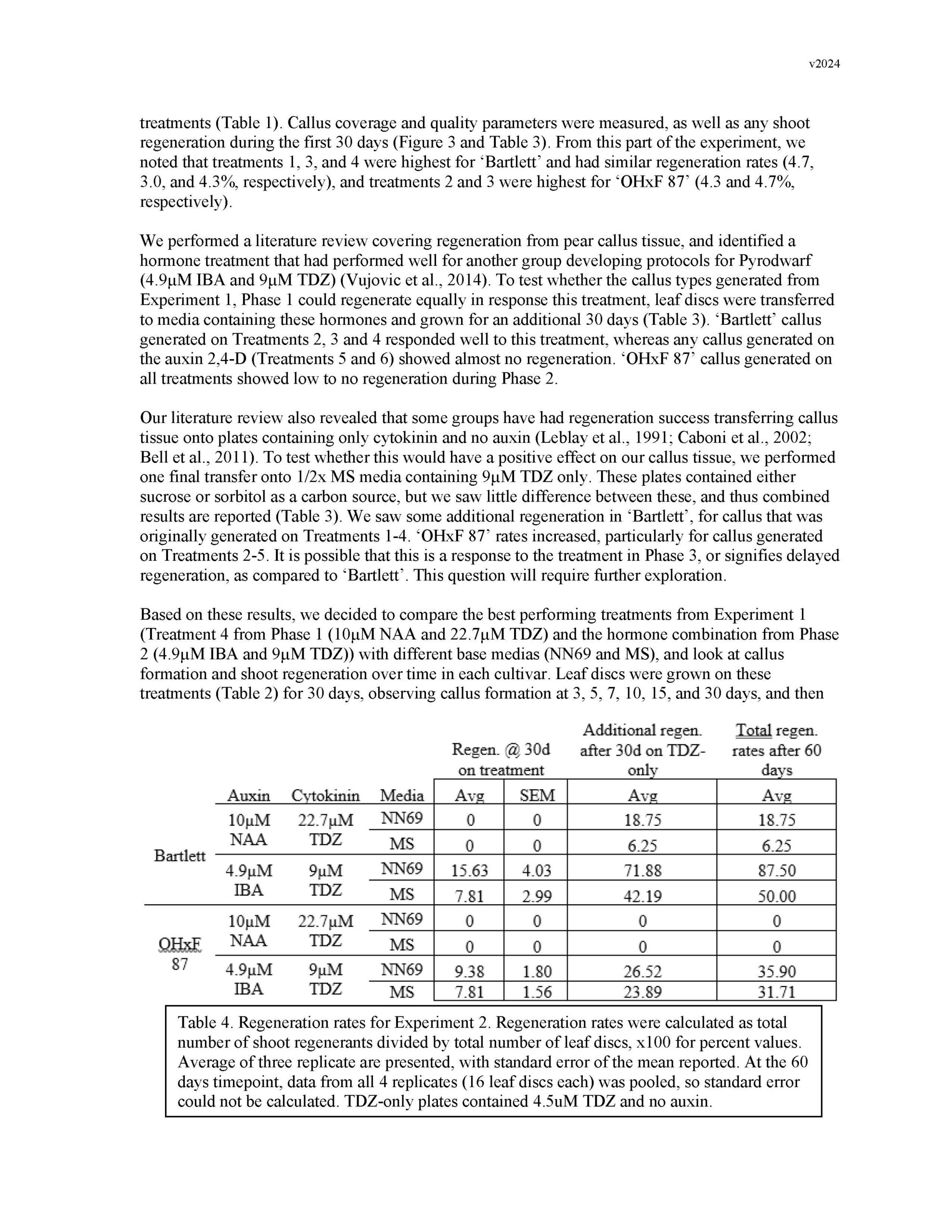
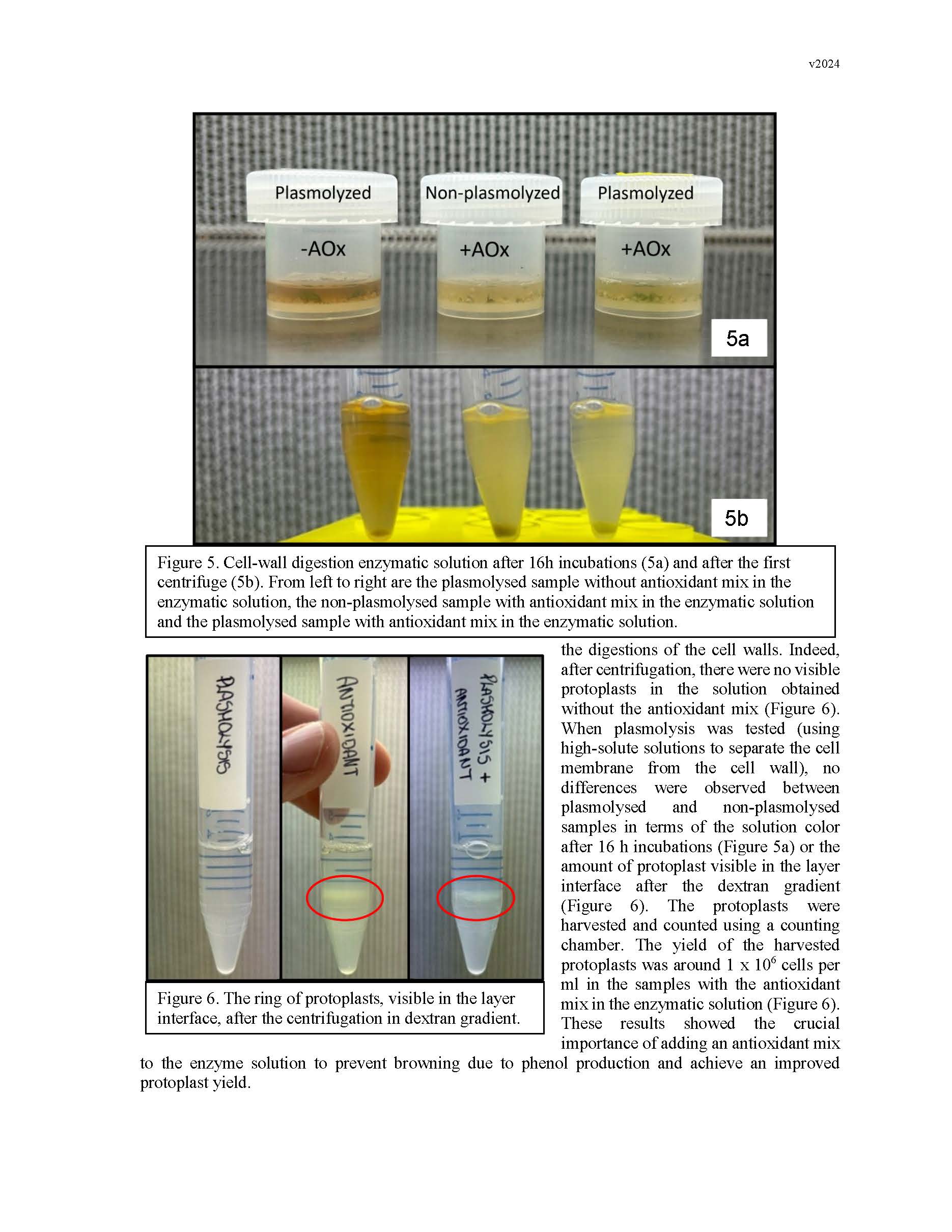
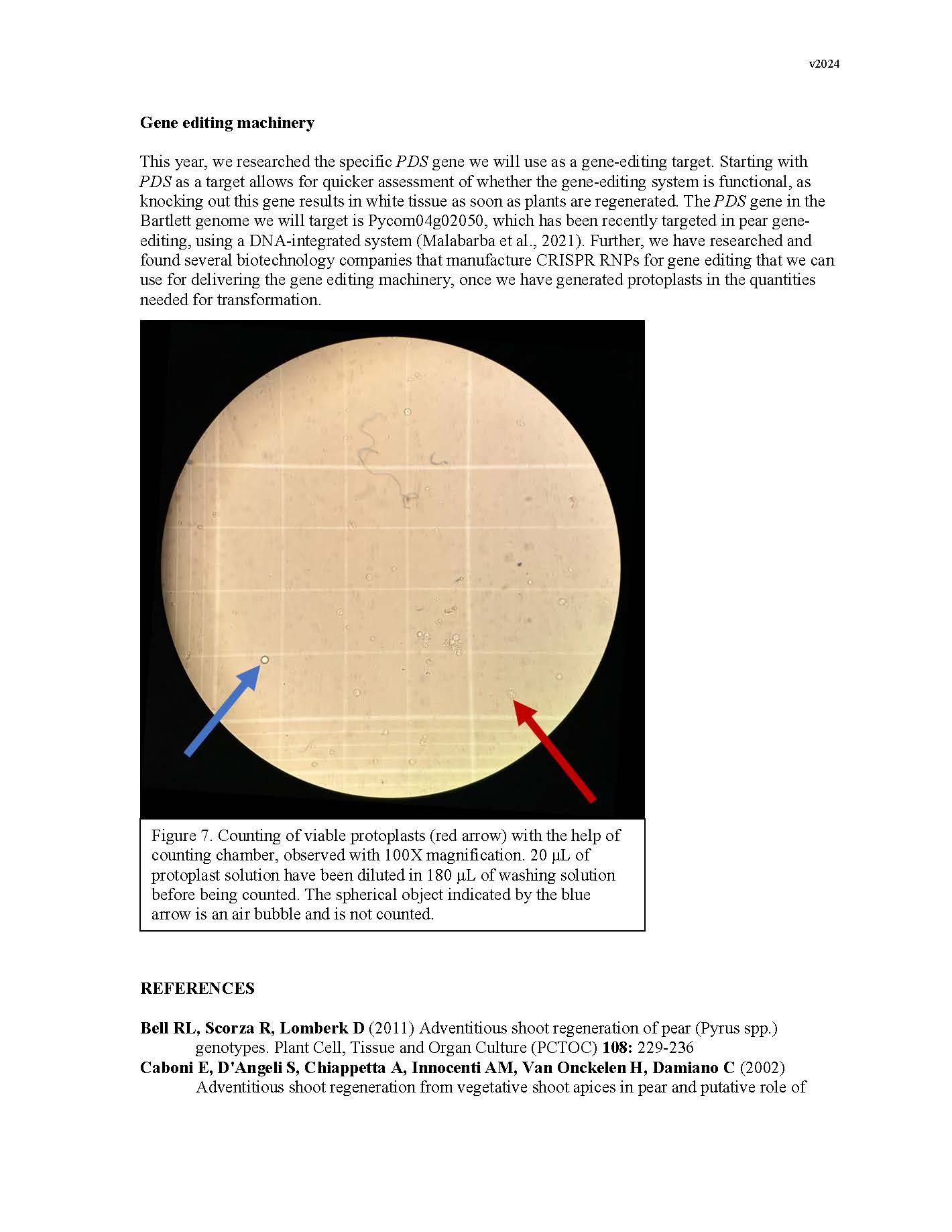
Summary: Gene editing has a strong potential to be useful for clonal crop species like pears. This is in part because it allows for the ability to make precise DNA changes without breeding, which gives us an additional tool for introducing traits into the germplasm. However, traditional gene-editing relies on the integration of transgenes into the plant's genome. Methods for the removal of transgenes often require additional rounds of breeding, especially for clonal species, which counteracts many of the benefits. In the past decade, researchers have begun developing methods for transgene-free gene editing in many crop plants, in which gene-editing machinery is introduced into plant cells without integrating any foreign genetic material into the plant's DNA. This reduces the need for additional rounds of breeding to address regulatory concerns. This year, we proposed to lay the groundwork for developing a transgene-free gene editing system in pears. To do this, we focused on optimizing adventitious shoot regeneration from pear callus tissue, began optimization of protoplast isolation from pear tissues, and researched gene targets and synthesis of gene editing machinery. Adventitious shoot regeneration from pear callus tissue was important for two reasons: allowing us to define a protocol for generating callus tissue that is competent to regenerate, and understanding the ideal hormone combinations each cultivar responds to for efficient regeneration. These will help us both to generate tissue for protoplast isolation and to regenerate plants from protoplasts. This year, we were able to screen different hormone combinations and identify an efficient protocol for 'Bartlett' callus formation and adventitious shoot regeneration. While we were able to increase efficiency slightly for OHxF 87 and 97, our future work will focus on improving efficiency for these cultivars. The two collaborating labs were able to meet this year and share methods, such that both groups have now begun the work of optimizing protoplast isolation. Our attempts thus far have narrowed the cell-wall digestion lengths but have struggled with partial digestion or oxidation issues. Future work will focus on testing different cell-wall digestion enzymes and concentrations, solution characteristics, and tissue sources. Finally, we identified the specific pear PDS gene and genetic sites to be targeted, as well as researched companies that can synthesize the RNPs we will use to introduce the gene-editing machinery into plant cells. All together, we took significant steps towards developing a transgene-free gene-editing system for pears and will continue working towards building this tool. Keywords: adventitious shoot regeneration, protoplasts, DNA-free transformation
Keywords: