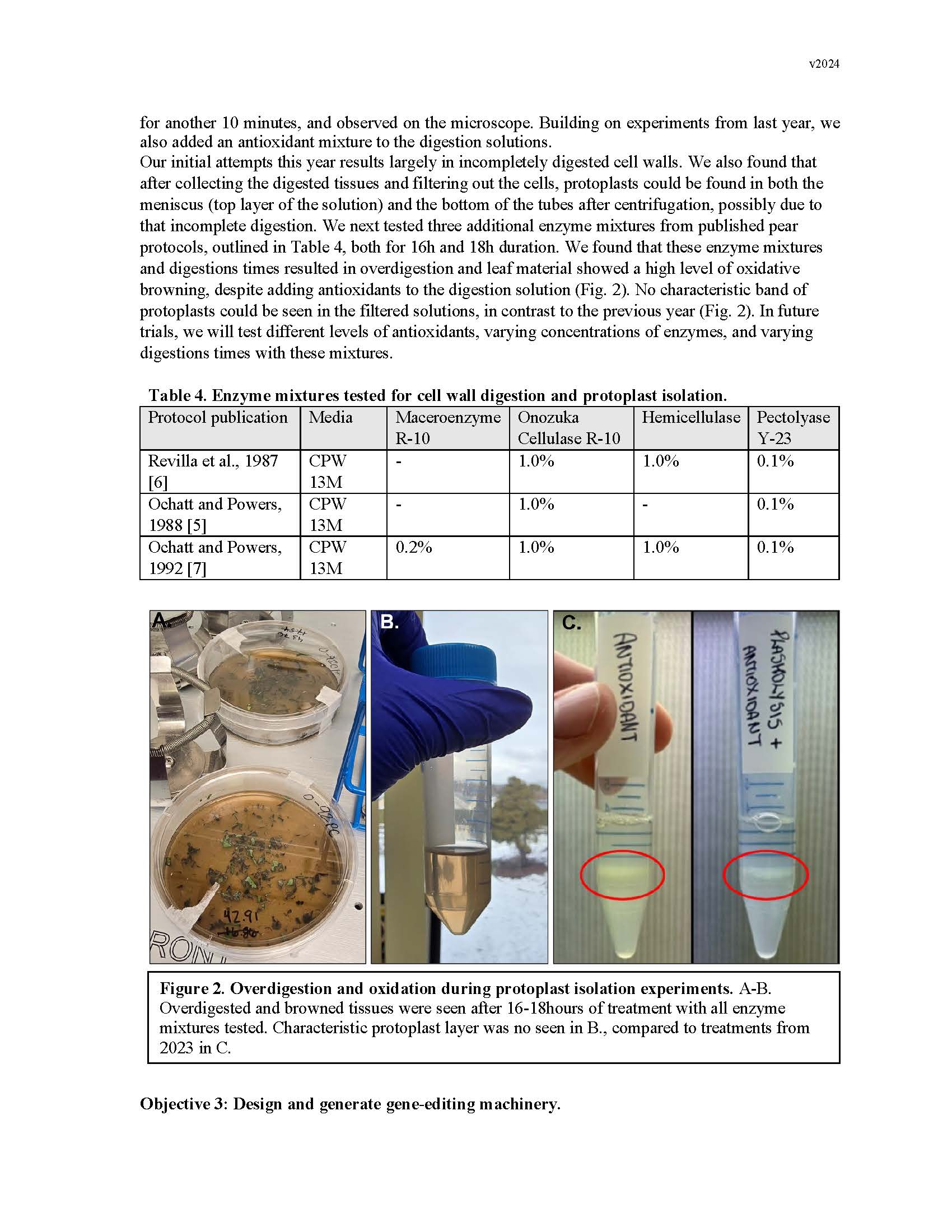
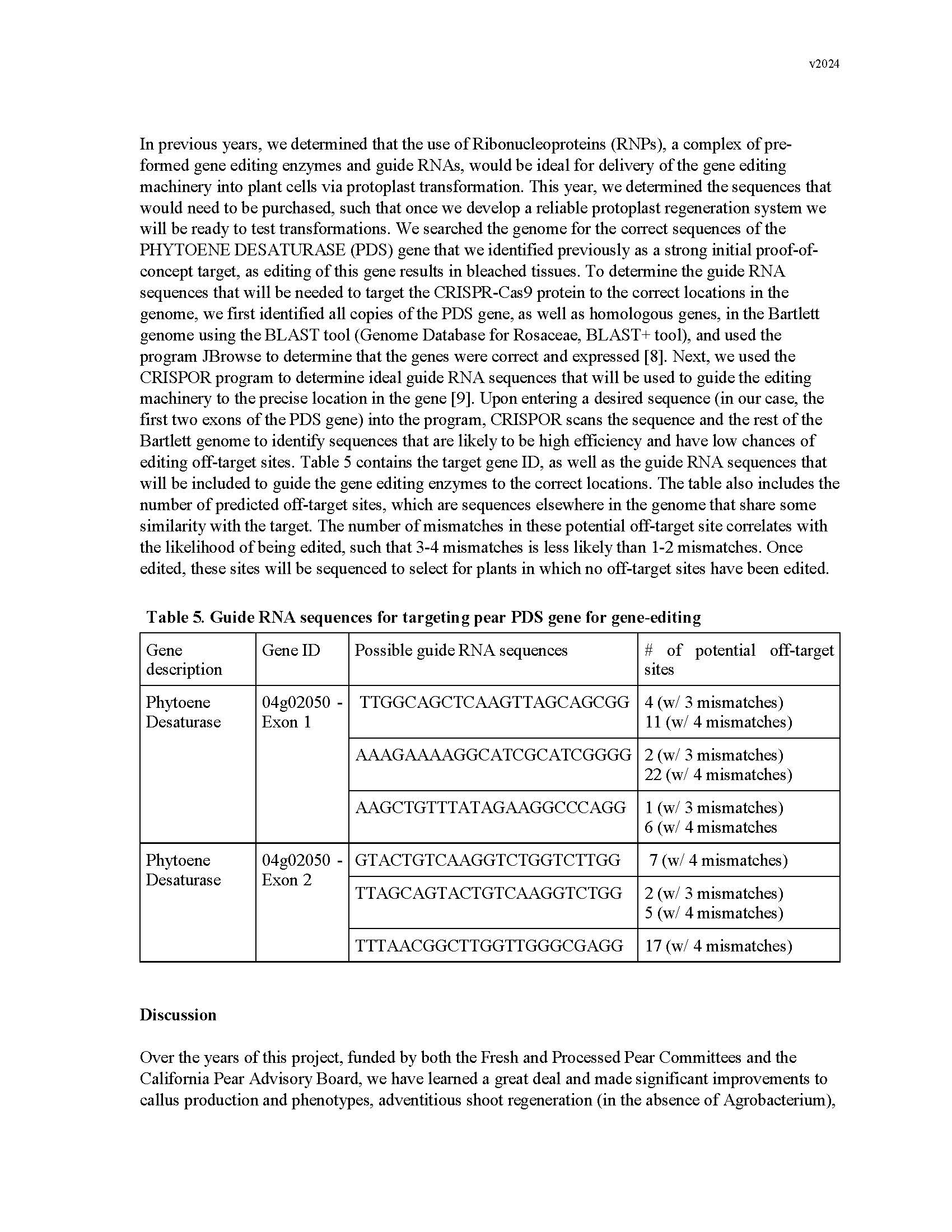
Development of a transgene-free gene editing system in European Pear
Author: Jessica Waite
Published: 2025
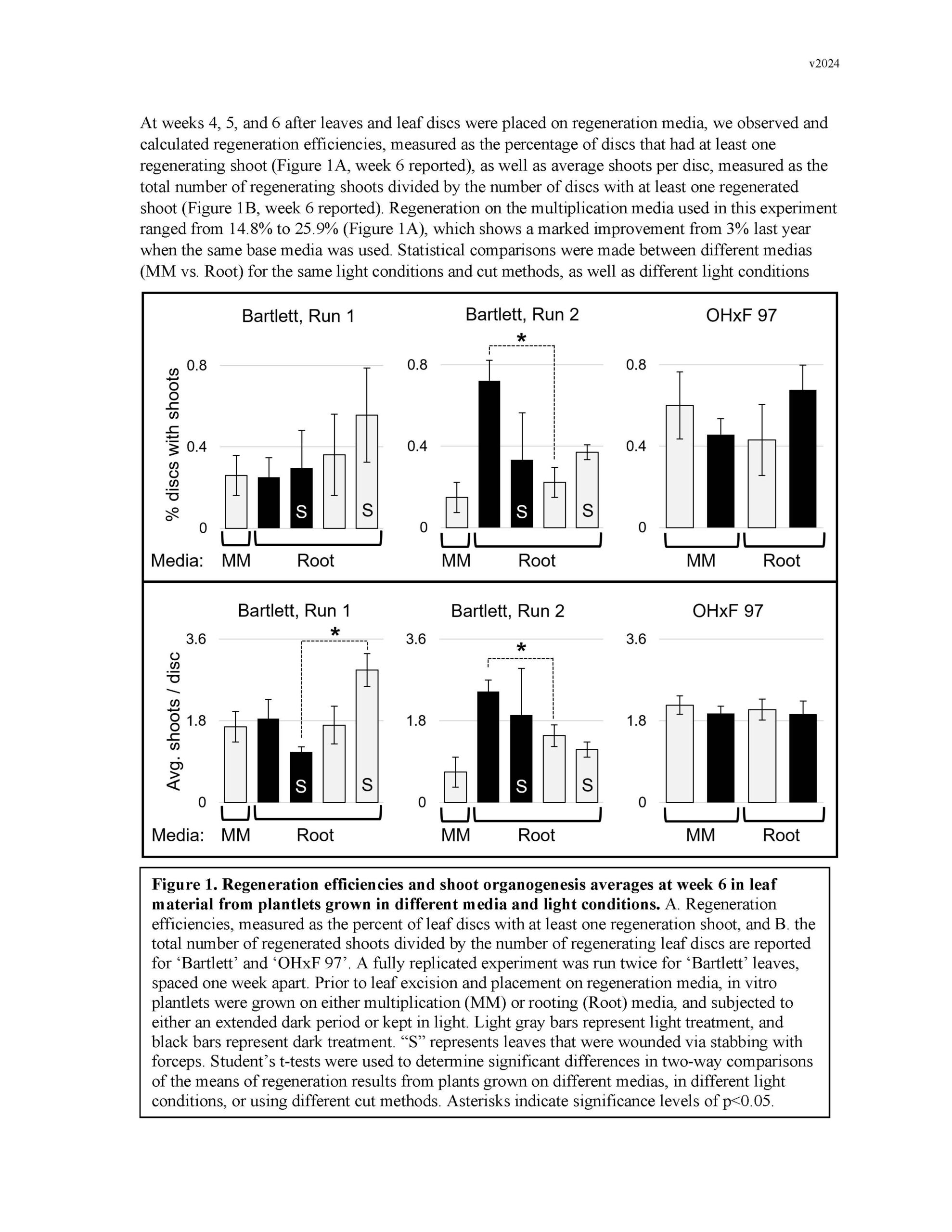
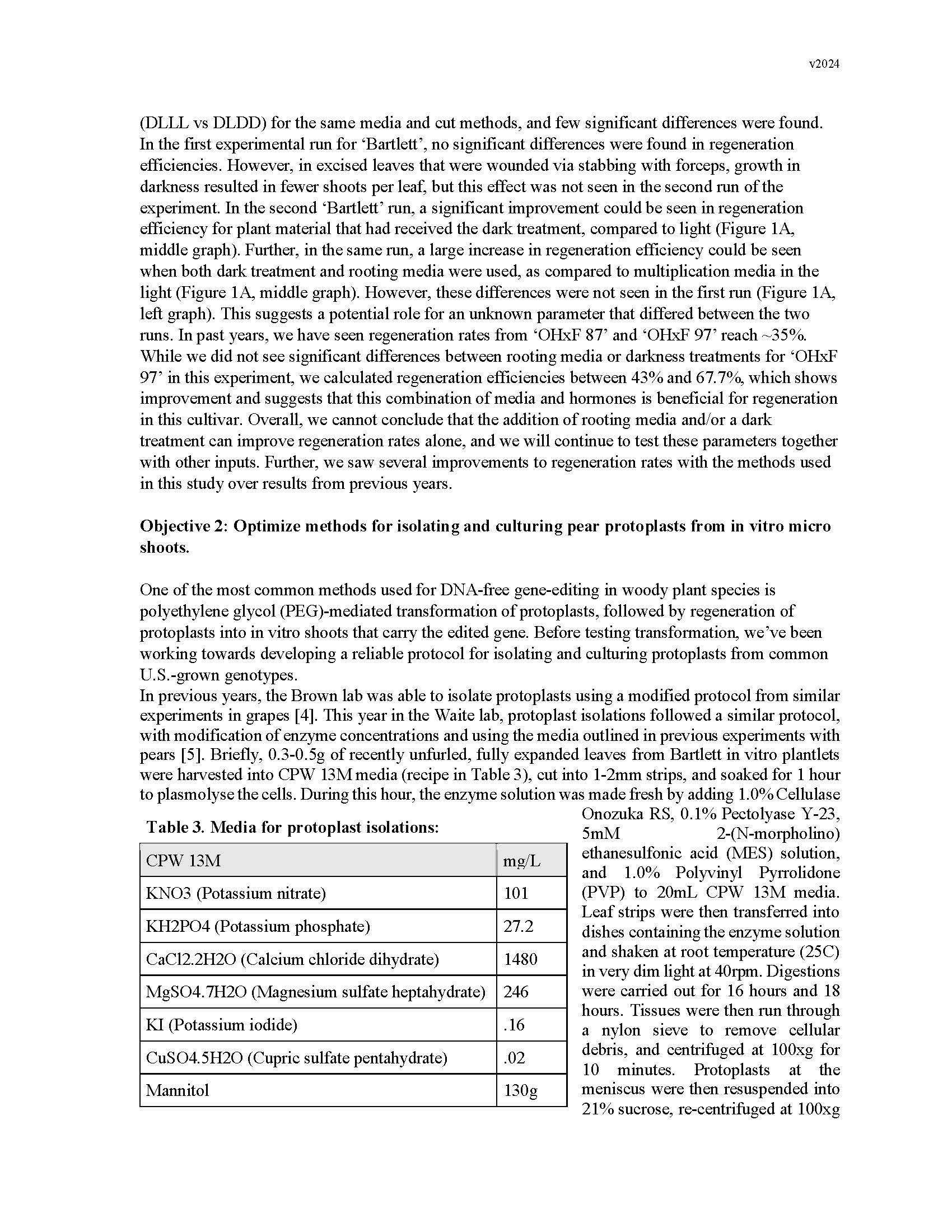
Summary: Gene editing has a strong potential to be useful for clonal crop species like pears. This is in part because it allows for the ability to make precise DNA changes without breeding, which gives us an additional tool for introducing traits into the germplasm. However, traditional gene-editing relies on the integration of transgenes into the plant's genome. Methods for the removal of transgenes often require additional rounds of breeding, especially for clonal species, which counteracts many of the benefits. In the past decade, researchers have begun developing methods for transgene-free gene editing in many crop plants, in which gene-editing machinery is introduced into plant cells without integrating any foreign genetic material into the plant's DNA. This reduces the need for additional rounds of breeding to address regulatory concerns. One of the most common ways this has been achieved is through introducing gene-editing machinery into protoplasts cells to edit the DNA, allowing these edited cells to grow into callus tissue, and then subsequently regenerating adventitious shoots from those cells. Protoplasts are plant cells which have had their cell walls digested, allowing for easier movement of the gene-editing machinery into the cell to reach the nucleus. The most difficult steps in this process are isolation of protoplasts and culture into callus, and adventitious shoot regeneration from that callus. This is in part due to each pear genotype having specific and distinct responses to media additives like nutrients and hormones. This year, we aimed to improve upon adventitious shoot regeneration protocols for ‘Bartlett’, ‘OHxF 87’ and ‘OHxF 97’ genotypes, optimize protoplast isolations by testing more digestion parameters, and finish designing the gene editing machinery. We focused in on one of our highest-producing shoot regeneration experiments from the previous year – plant growth on rooting media and dark treatment prior to leaf excision – and found that when performed on a larger scale, these treatments alone did not improve adventitious shoot regeneration. However, our efficiency rates were generally higher than they have been in the past, signifying that overall, our base protocols have improved. Protoplast isolation trials were run to test enzyme concentrations, combinations, and digestion times from three previously-published protocols for pears, and all led to overdigestion and oxidation. In future trials, we will expand the concentrations and digestion times with these enzymes, as well as vary the antioxidant concentrations in the media. Finally, specific guide-RNA target sequences of the Phytoene Desaturase gene were identified to be used with the gene-editing machinery once a protoplasts isolation and regeneration system are established. Future work will continue to focus on developing and optimizing protocols for these more difficult steps, such that genotypes important for the U.S. pear industry can be edited for important trait-associated genes.
Keywords: